Effective Disinfection: A Core Pillar of Cleanroom Contamination Control
 BlogCleanroom07.10.2025
BlogCleanroom07.10.2025
Maintaining contamination control is central to the integrity and reliability of cleanroom operations. At the heart of this process lies effective disinfection. Thorough and validated cleaning and disinfection is not optional; it is a fundamental requirement under EU GMP Annex 1.
Why Cleaning Must Come Before Disinfection
Disinfection can only work effectively if preceded by proper cleaning. Cleaning removes surface contamination such as dust, dirt, and residues, allowing the disinfectant to make direct contact with microorganisms. Without this vital step, the disinfectant’s efficacy is reduced, potentially leaving contamination risks behind.
Regulatory Requirements
EU GMP Annex 1 Sections 4.32 to 4.36 set out clear expectations for cleanroom disinfection programmes, requiring:
- Cleaning before disinfection to remove surface contamination.
- Multiple disinfectants with different modes of action to so that their combined usage is effective against a wide range of bacteria and fungi.
- Periodic use of a sporicidal agent to target resilient spores.
- Initial validation followed by ongoing monitoring to confirm continued effectiveness
These requirements ensure that disinfection strategies are both robust and sustainable over time.
Building an Effective Disinfection Regime
To align with regulatory expectations and deliver consistent results, Micronclean recommend a rotational cleaning and disinfection programme using our Delta, Alpha, Beta, and Zyceine mopping systems:
- Delta, A neutral detergent designed to effectively remove surface contamination
- Alpha, A broad-spectrum disinfectant
- Beta, A second broad-spectrum disinfectant with a different mode of action
- Zyceine, A highly effective sporicide, used periodically to supplement routine disinfection
Micronclean Recommended Regime:
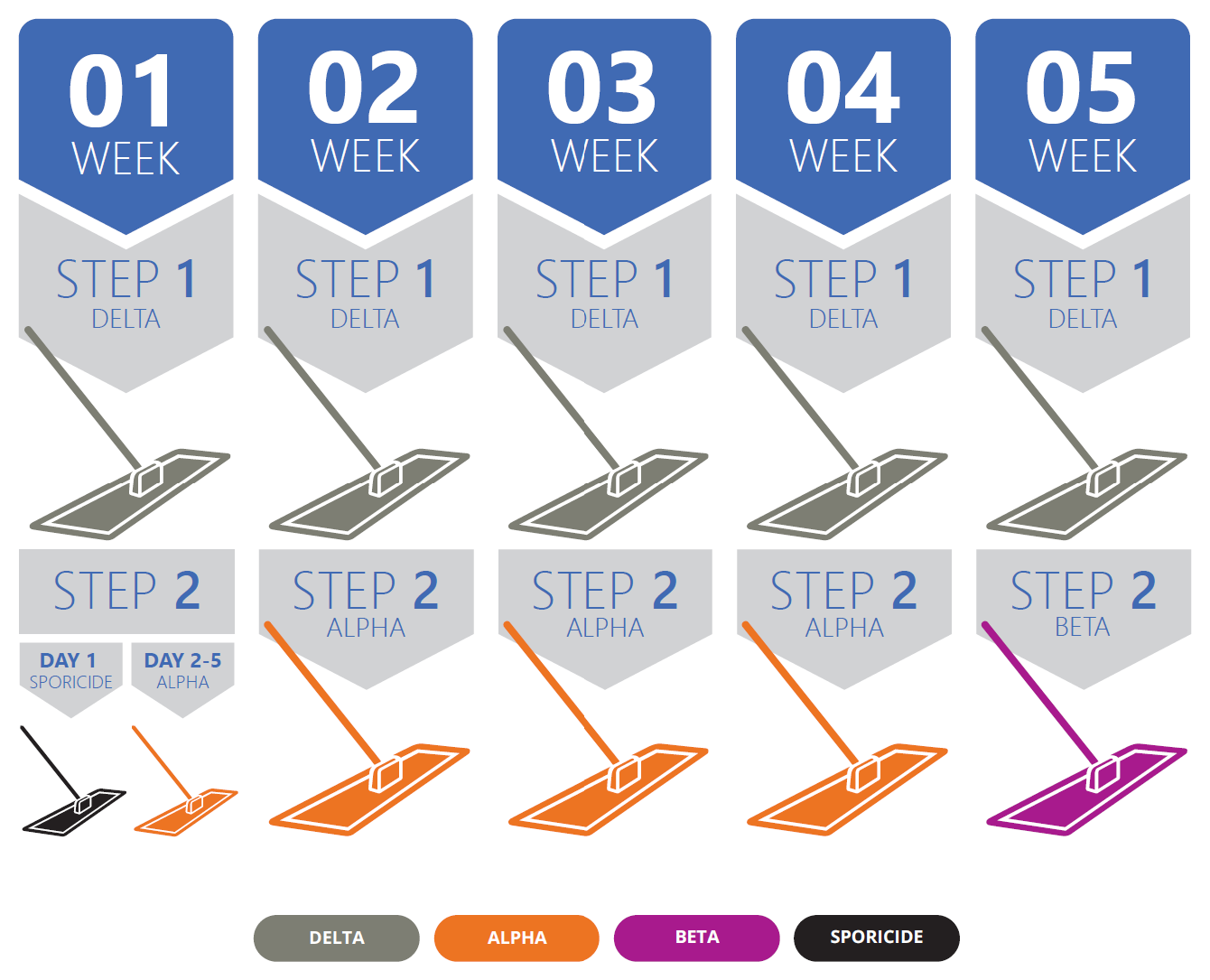
Week 1 | Day 1
Clean the floor using Delta Mops followed by the use of a Sporicide Mop following the manufacturer’s instruction.
Week 1 | Day 2–5
Clean the floor by using Delta Mops followed by disinfection using Alpha Mops.
Weeks 2 to 4 | Every Day
As in Week 1 Days 2–5, clean the floor by using Delta Mops followed by disinfection using Alpha Mops.
Week 5 | Every Day
As in the previous week, clean the floor using Delta Mops but then use a Beta Mop as the rotational disinfection step.
This structured four-step rotation provides:
- Compliance with EU GMP Annex 1
- Maximum efficacy against a wide range of microorganisms
- Cost-effective protection while minimising corrosion risks
Balancing Efficacy with Safety
While sporicides, like Zyceine are extremely effective, excessive use can increase the risk of corrosion and occupational exposure. By incorporating Zyceine strategically into a rotational system, you achieve strong microbial control without unnecessary damage to equipment or risks to personnel.
Continuous Testing and Validation
No matter how robust the regime, every disinfection programme must be validated within your own cleanroom environment. Continuous monitoring of microbial load and surface contamination ensures compliance, maintains safety, and confirms that the chosen system remains effective over time.
Conclusion
Effective disinfection is a cornerstone of cleanroom contamination control. By following Annex 1 guidance, implementing a rotational disinfection programme, and continuously monitoring results, cleanroom operators can ensure both regulatory compliance and long-term protection of processes, products and people.

Author
Tasmin Crompton
Technical Lead
